ChargeCalculator:Case studies: Difference between revisions
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
We present a few interesting examples of use cases for '''ACC'''. | We present a few interesting examples of use cases for '''ACC'''. | ||
=Paracetamol | =Paracetamol – atomic charges and chemical reactivity in small drug like molecules= | ||
[[File:Paracetamol.png|thumb|right|650px|The most positive H in the paracetamol molecule correspond to the most reactive sites during the metabolic degradation of paracetamol.]] | |||
N-acetyl-p-aminophenol, commonly known as paracetamol, is a widely used analgesic and antipyretic. Its mechanism of action is believed to be the inhibition of the protein cyclooxygenase 2, regulating the production of pro-inflammatory compounds. The metabolic break down of paracetamol has been the subject of intense study, since it holds the key to both its therapeutic action and toxicity. | |||
The | We calculated atomic charges in paracetamol using ACC. The geometry of the paracetamol molecule corresponded to the ideal coordinates for the entry TYL from the wwPDB Chemical Compounds Database (wwPDB CCD: TYL). The default ACC settings were used. The computation took less than 1s, and the complete results are available on the [http://ncbr.muni.cz/ACC/CaseStudy/Paracetamol ACC web page]. | ||
A quick analysis reveals that the phenolic H (position HO4) is the most acidic proton (highest positive charge) in the molecule, suggesting a faster and easier dissociation of this O-H bond. Indeed up to 90% of metabolic degradation happens at position HO4 (glucunronidationration, sulphonation). Additionally, up to 15% of metabolic degradation involves dehydrogenation oxidation at the phenolic (HO4) and amidic positions (HN), the two most positive H in the paracetamol molecule. While paracetamol is a very small molecule with few polar sites, the same principle can be applied in reasoning out highly reactive sites in more complex molecules. | |||
You can try out this particular computation setup [http://webchem.ncbr.muni.cz/Platform/ChargeCalculator/Result/8ed40f3c-48e7-4a1d-9d86-7ec541c95984?example=Paracetamol here], and make sure to use the interactive guides for additional explanations. We have used the default ACC settings, meaning that the total charge was 0, the EEM parameter set covers NPA atomic charges at the B3LYP/6-31G* level of theory. These charges are expected to support qualitative chemical concepts like induction and conjugation. In general, it is a good idea to try several charge definitions. One or more of these might prove useful in further modeling studies. If you plan to run molecular dynamics, it is good to use charges which are compatible with the particular force field you plan to use. For instance, if the force field is known to use atomic charges based on electrostatic potential mapping, pick at least one EEM parameter set developed with this charge definition. | |||
<br style="clear:both" /> | |||
= | =Protegrin-1 – atomic charges and activity of antimicrobial peptides= | ||
[[File:Protegrin.png|thumb|right|300px| Residue centroids represented by spheres colored by residue charges. Residues ARG4 and ARG9 are significantly less positive (lighter blue) than the rest of the ARG residues (darker blue), indicating that mutations at these positions may be less effective.]] | |||
Protegrins are a family of antimicrobial peptides active against a wide range of pathogens. Protegrin-1 (PG1) has been intensely studied for its potential in treating infections caused by antibiotic resistant bacteria. PG1 shows activity against several pathogens, but also toxicity against the host. Useful mutations are those which maintain the antimicrobial activity, and at the same time reduce toxicity. | |||
We calculated atomic charges in PG1 using ACC. The geometry of the PG1 molecule corresponded to the lowest energy NMR model in the entry 1PG1 (PDB: 1PG1) from the Protein Data Bank. The computation took less than 1s, and the complete results are available on the [http://ncbr.muni.cz/ACC/CaseStudy/Protegrin ACC web page]. | |||
The calculation produced one set of atomic charges. PG1 contains 18 residues, and rather than analyzing atomic charges, we analyzed the residue charges, which are also reported by ACC (Figure 4B). PG1 is special because of its high positive charge. It contains 6 ARG residues. However, not all have the same charge. In particular, ARG at positions 4 and 9 have the least positive charge (around +0.5e), whereas the rest have much higher positive charge (over +0.8e). Such results suggest that mutations of ARG into a neutral residue at positions 4 or 9 would have a lower effect than mutations at positions 1, 10, 11 or 18. This seems to be indeed the case, as it was found that the mutation of ARG 4 alters the antimicrobial activity against C. albicans significantly less than the mutation of ARG 10. Such biologically relevant insight can be gained by analyzing the residue charges on a single structure of PG1. | |||
You can try out this particular computation setup [http://webchem.ncbr.muni.cz/Platform/ChargeCalculator/Result/dc41612f-b3c0-4207-97a6-b70598e90d88?example=Protegrin here], and make sure to use the interactive guides for additional explanations. The total molecular charge was +7, owing to the many ARG residues. The EEM parameter set used was EX-NPA_6-31Gd_gas, but it was necessary to add to this set EEM parameters for deuterium (D), because this element was present in the input file. The EEM parameters for D were identical to the parameters for H. The EEM parameter set covers NPA atomic charges at the HF/6-31G* level of theory. These charges are expected to support qualitative chemical concepts like induction and conjugation. The rest of the ACC default settings were kept. | |||
<br style="clear:both" /> | |||
=Paracetamol – atomic charges and allostery of large biomacromolecular complexes= | |||
[[File:Proteasome.png|thumb|right|650px|The 26S proteasome is a large machine consisting of a regulatory particle and a core particle. The core particle is made up of alpha and beta rings. Information pertaining to allosteric regulation travels across the proteasome in a vertical and horizontal fashion, and can be traced based on the change in total charge between different conformational states.]] | |||
The 26S proteasome is a large biomacromolecular complex which facilitates the targeted degradation of intracellular proteins, and thus plays an essential role in keeping protein homeostasis. It consists of a core particle, made up of alpha rings and beta rings, controlled by regulatory particles, which are made up of a number of proteins. The proteasome is an intricate molecular machine which requires complex regulation to unfold and deubiquityilate the substrate, and push it through the catalytic machinery located in the beta rings. Necessarily, the proteasome undergoes large conformational changes during its operation. However, due to its size, such changes are very difficult to study. Recent work in the field of cryo-electron microscopy has lead to the discovery of intermediate conformers during the initial binding of ubiquitylated substrates. While the conformational changes in the regulatory particle are easily distinguishable (average backbone atom RMSD 10.4 Å), the changes in the core particle are very subtle (average backbone atom RMSD 1.5 Å), due to the fact that all studied conformers refer to the initial phase of substrate binding. | |||
Using ACC, we calculated atomic charges in these 3 intermediate conformers of the 26S proteasome that are available in the Protein Data Bank (PDB IDs: 4CR2, 4CDR3, 4CR4). The default ACC settings were used. The computation took 130s, and the complete results are available [http://ncbr.muni.cz/ACC/CaseStudy/Proteasome on the ACC web page]. | |||
The | The calculation produced one 3 sets of atomic charges for each conformer of the 26S proteasome. Since the proteasome is very large, it is difficult to draw conclusions based only on atomic or even residue charges. To obtain the total charge for a specific subunit or even entire particle, just sum up all the atomic charges for atoms which belong to that subunit or particle. Subunit information can be found in the input file in the form of chains. Thus, atoms with the chain identifier 1-7 belong to the beta ring, A-G belong to the alpha ring, and H-Z belong to the regulatory particle. By comparing the total charge of each particle or subunit across the conformational state 1 (PDB ID: 4CR2), state 2 (PDB ID: 4CR3), and state 3 (PDB ID: 4CR4), conclusions can be drawn regarding the direction of information flow inside the proteasome. | ||
The | The first observation is that, during the conformational changes from state 1, to state 2, and then to state 3, a significant amount of electron density is transferred between the core particle and the regulatory particle. This suggests that, even though there is no significant movement observed for the core particle, allosteric information is exchanged with the core particle, and this information can be tracked at the electrostatic level. | ||
The next observation is that significant information is disseminated not only horizontally (within the alpha ring, or within the beta ring), but also vertically. In the overall transition it appears that the alpha ring loses electron density to the regulatory particle. By checking the intermediate state 2 it is possible to see that there is also transfer between the alpha ring and beta ring. This vertical shuttling of electron density within the catalytic core particle suggests that the activity of alpha and beta subunits may cross-correlate. Such phenomena have indeed been reported. For example, alpha5 and beta1 may translocate together, while knockdown of alpha1 leads to loss of chymotrypsin activity associated with beta5. | |||
Further analysis can even yield the residues involved in the allosteric regulation, as those residues which exhibit a high variation in total charge (e.g., approximately 10 sites on the Rpn-13 regulatory subunit). This case study shows how a brief calculation using only a crude structural approximation can give insight regarding allosteric regulation in large biomolecular complexes. | |||
You can try out this particular computation setup [http://webchem.ncbr.muni.cz/Platform/ChargeCalculator/Result/47a5bd67-5b9f-4138-8432-c1b9dc059b8e?example=Proteasome here], and make sure to use the interactive guides for additional explanations. We have used the default ACC settings, meaning that the total charge was 0 for all conformers, the EEM parameter set covers NPA atomic charges at the HF/6-31G* level of theory. These charges are expected to support qualitative chemical concepts like induction and conjugation. In general, it is a good idea to try several charge definitions. One or more of these might prove useful in further modeling studies. If you plan to run molecular dynamics, it is good to use charges which are compatible with the particular force field you plan to use. For instance, if the force field is known to use atomic charges based on electrostatic potential mapping, pick at least one EEM parameter set developed with this charge definition. | |||
<br style="clear:both" /> | |||
Latest revision as of 19:14, 14 June 2015
We present a few interesting examples of use cases for ACC.
Paracetamol – atomic charges and chemical reactivity in small drug like molecules
[edit]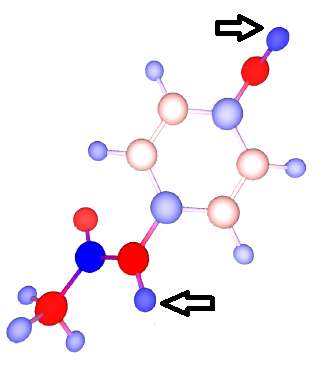
N-acetyl-p-aminophenol, commonly known as paracetamol, is a widely used analgesic and antipyretic. Its mechanism of action is believed to be the inhibition of the protein cyclooxygenase 2, regulating the production of pro-inflammatory compounds. The metabolic break down of paracetamol has been the subject of intense study, since it holds the key to both its therapeutic action and toxicity.
We calculated atomic charges in paracetamol using ACC. The geometry of the paracetamol molecule corresponded to the ideal coordinates for the entry TYL from the wwPDB Chemical Compounds Database (wwPDB CCD: TYL). The default ACC settings were used. The computation took less than 1s, and the complete results are available on the ACC web page.
A quick analysis reveals that the phenolic H (position HO4) is the most acidic proton (highest positive charge) in the molecule, suggesting a faster and easier dissociation of this O-H bond. Indeed up to 90% of metabolic degradation happens at position HO4 (glucunronidationration, sulphonation). Additionally, up to 15% of metabolic degradation involves dehydrogenation oxidation at the phenolic (HO4) and amidic positions (HN), the two most positive H in the paracetamol molecule. While paracetamol is a very small molecule with few polar sites, the same principle can be applied in reasoning out highly reactive sites in more complex molecules.
You can try out this particular computation setup here, and make sure to use the interactive guides for additional explanations. We have used the default ACC settings, meaning that the total charge was 0, the EEM parameter set covers NPA atomic charges at the B3LYP/6-31G* level of theory. These charges are expected to support qualitative chemical concepts like induction and conjugation. In general, it is a good idea to try several charge definitions. One or more of these might prove useful in further modeling studies. If you plan to run molecular dynamics, it is good to use charges which are compatible with the particular force field you plan to use. For instance, if the force field is known to use atomic charges based on electrostatic potential mapping, pick at least one EEM parameter set developed with this charge definition.
Protegrin-1 – atomic charges and activity of antimicrobial peptides
[edit]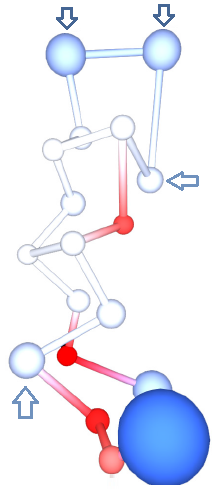
Protegrins are a family of antimicrobial peptides active against a wide range of pathogens. Protegrin-1 (PG1) has been intensely studied for its potential in treating infections caused by antibiotic resistant bacteria. PG1 shows activity against several pathogens, but also toxicity against the host. Useful mutations are those which maintain the antimicrobial activity, and at the same time reduce toxicity.
We calculated atomic charges in PG1 using ACC. The geometry of the PG1 molecule corresponded to the lowest energy NMR model in the entry 1PG1 (PDB: 1PG1) from the Protein Data Bank. The computation took less than 1s, and the complete results are available on the ACC web page.
The calculation produced one set of atomic charges. PG1 contains 18 residues, and rather than analyzing atomic charges, we analyzed the residue charges, which are also reported by ACC (Figure 4B). PG1 is special because of its high positive charge. It contains 6 ARG residues. However, not all have the same charge. In particular, ARG at positions 4 and 9 have the least positive charge (around +0.5e), whereas the rest have much higher positive charge (over +0.8e). Such results suggest that mutations of ARG into a neutral residue at positions 4 or 9 would have a lower effect than mutations at positions 1, 10, 11 or 18. This seems to be indeed the case, as it was found that the mutation of ARG 4 alters the antimicrobial activity against C. albicans significantly less than the mutation of ARG 10. Such biologically relevant insight can be gained by analyzing the residue charges on a single structure of PG1.
You can try out this particular computation setup here, and make sure to use the interactive guides for additional explanations. The total molecular charge was +7, owing to the many ARG residues. The EEM parameter set used was EX-NPA_6-31Gd_gas, but it was necessary to add to this set EEM parameters for deuterium (D), because this element was present in the input file. The EEM parameters for D were identical to the parameters for H. The EEM parameter set covers NPA atomic charges at the HF/6-31G* level of theory. These charges are expected to support qualitative chemical concepts like induction and conjugation. The rest of the ACC default settings were kept.
Paracetamol – atomic charges and allostery of large biomacromolecular complexes
[edit]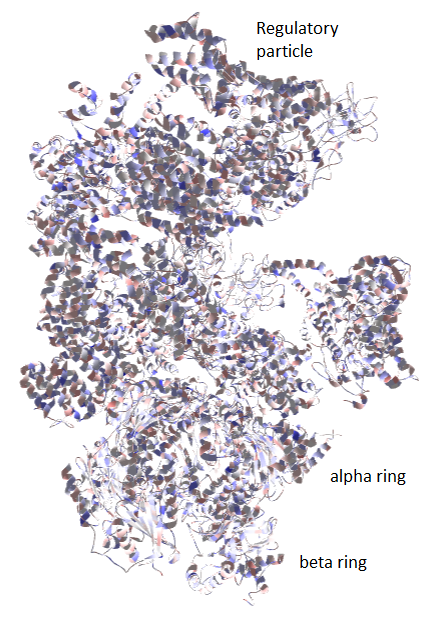
The 26S proteasome is a large biomacromolecular complex which facilitates the targeted degradation of intracellular proteins, and thus plays an essential role in keeping protein homeostasis. It consists of a core particle, made up of alpha rings and beta rings, controlled by regulatory particles, which are made up of a number of proteins. The proteasome is an intricate molecular machine which requires complex regulation to unfold and deubiquityilate the substrate, and push it through the catalytic machinery located in the beta rings. Necessarily, the proteasome undergoes large conformational changes during its operation. However, due to its size, such changes are very difficult to study. Recent work in the field of cryo-electron microscopy has lead to the discovery of intermediate conformers during the initial binding of ubiquitylated substrates. While the conformational changes in the regulatory particle are easily distinguishable (average backbone atom RMSD 10.4 Å), the changes in the core particle are very subtle (average backbone atom RMSD 1.5 Å), due to the fact that all studied conformers refer to the initial phase of substrate binding.
Using ACC, we calculated atomic charges in these 3 intermediate conformers of the 26S proteasome that are available in the Protein Data Bank (PDB IDs: 4CR2, 4CDR3, 4CR4). The default ACC settings were used. The computation took 130s, and the complete results are available on the ACC web page.
The calculation produced one 3 sets of atomic charges for each conformer of the 26S proteasome. Since the proteasome is very large, it is difficult to draw conclusions based only on atomic or even residue charges. To obtain the total charge for a specific subunit or even entire particle, just sum up all the atomic charges for atoms which belong to that subunit or particle. Subunit information can be found in the input file in the form of chains. Thus, atoms with the chain identifier 1-7 belong to the beta ring, A-G belong to the alpha ring, and H-Z belong to the regulatory particle. By comparing the total charge of each particle or subunit across the conformational state 1 (PDB ID: 4CR2), state 2 (PDB ID: 4CR3), and state 3 (PDB ID: 4CR4), conclusions can be drawn regarding the direction of information flow inside the proteasome.
The first observation is that, during the conformational changes from state 1, to state 2, and then to state 3, a significant amount of electron density is transferred between the core particle and the regulatory particle. This suggests that, even though there is no significant movement observed for the core particle, allosteric information is exchanged with the core particle, and this information can be tracked at the electrostatic level.
The next observation is that significant information is disseminated not only horizontally (within the alpha ring, or within the beta ring), but also vertically. In the overall transition it appears that the alpha ring loses electron density to the regulatory particle. By checking the intermediate state 2 it is possible to see that there is also transfer between the alpha ring and beta ring. This vertical shuttling of electron density within the catalytic core particle suggests that the activity of alpha and beta subunits may cross-correlate. Such phenomena have indeed been reported. For example, alpha5 and beta1 may translocate together, while knockdown of alpha1 leads to loss of chymotrypsin activity associated with beta5.
Further analysis can even yield the residues involved in the allosteric regulation, as those residues which exhibit a high variation in total charge (e.g., approximately 10 sites on the Rpn-13 regulatory subunit). This case study shows how a brief calculation using only a crude structural approximation can give insight regarding allosteric regulation in large biomolecular complexes.
You can try out this particular computation setup here, and make sure to use the interactive guides for additional explanations. We have used the default ACC settings, meaning that the total charge was 0 for all conformers, the EEM parameter set covers NPA atomic charges at the HF/6-31G* level of theory. These charges are expected to support qualitative chemical concepts like induction and conjugation. In general, it is a good idea to try several charge definitions. One or more of these might prove useful in further modeling studies. If you plan to run molecular dynamics, it is good to use charges which are compatible with the particular force field you plan to use. For instance, if the force field is known to use atomic charges based on electrostatic potential mapping, pick at least one EEM parameter set developed with this charge definition.